Division of Nuclear Medicine
Staff
The task of the Division
Educated stuff of the Division and Experimental Animal House consist radiopharmacists, veterinarians, physicist, chemist, bio-engineer and nuclear medicine technicians, animal keepers with many years experience. Their activities are wide-scaling – basicly regarding to medicinal and veterinary isotope applications. Theire work involves preparing the governmental rules, writing the laboratory methodology of the topic, researching actual problems and the contimous postgradual education of the specialists in the field.
In the Nuclear Medicine Departments of Hungarian hospitals a couple of scintigraphical examinations is a daily routine as well. For performing these diagnostical procedures, most importantly needed the detecting instrument (gamma camera) and the brunch of diagnostical radiopharmaceuticals. There is a few dozens of radiopharmaceuticals for diagnostical purposes, most of them are 99mTechnetium labelled molecules e.g.: 99mTc MDP for bone scintigraphy, or 99mTc MIBI for heart perfision studies – but also in Hungary there is an increasing number of positron emission tomography (PET) examinations that is performed by the use of positron emitting radiopharmaceuticals e.g.: (18FDG and 14C-methyonin). Similarly to the international trends the use of radiopharmaceuticals in treatment protocols is also increasing in Hungary.
Nuclear medicine treatment methods are mainly used in cancerous patients but there are many orthopaedic and other diseases also treated by therapeutical radiopharmaceuticals. The best known and frequently used therapeutical radiopharmaceuticals are 153Samarium and 177Lutecium labelled phophonates (153Sm and 177Lu EDTMP) for bone metastases palliation, 131J-iodine for thyroid diseases, and 90Yttrium-colloids for radiosynovectomy in therapy resistant chronis arthritis patients.
Radiopharmaceutical application must be effective (sensitive and specific in the diagnosis and effective in the treatment) and paralelly fullfill all the requirements of safety. Applied radiopharmaceutical opens numerous chapters of public health: it must be harmless for the patients and their family, for the examining stuff (nuclear medicine technicians and physicians) and for the environment. The use of radiopharmaceuticals is operated by many governmental and european laws and rules that are contimously renewed, rewritten, and constructed for the ever changing practice and educated, communicated with the clinician specialists. These duties are addressed for the Division`s and Experimental Animal House workers.
- Radiopharmaceuticals given into the patient by different routes (intravenam, per os, subcutaneously, intratumorally, intraarticularly) distribute within the organism, concentrate in the target organs, while the extraproportion of applied radiopharmaceuticals leave the body via the urine, faeces and other excrets e.g.: saliva. The timing of distribution and excretion processes together with physical properties of the isotope (physical half-life, type and energy of radiation) determine the internal dosimetry data of the patient. Ideally, high target doses exist with low excretory or critical organ doses. Reaching this goal of clinicians radiopharmaceutical research and development on experimental animals is needed. Animal test`s data are extrapolated in humans using special softvers (MIRDOSE, OLINDA). Our experimental work on laboratory animals goals to minimize the radiation side effects and maximize effectivity of nuclear medicine diagnostical and therapeutical achivements in the human subjects.
- There are many ways in modifying patient internal dosimetry data so that higher target organ dose and lower critical organ doses could be reached. Telling the same in other words finding the maximum tolerable dose of a radiopharmaceutical is maybe the most important criteria of radiopharmaceutical application. Using animal models methods could be offered for blocking the thyroid glands, and decreasing the organ doses of the kidneys, liver, gastric mucosa and intestines or the bone marrow.
- There are only very limited data on examined or treated human patients regarding to the excreted proportion of radiopharmaceutical in milk of breast-feeding women and sperm. What time can women start to breast-feed their children? How long should men avoid from sexual interaction? Answering these questions animal test data are necessary for sure.
- An ever rising problem when human or veterinary diagnostical or therapeutical nuclear medicine applications are performed – do we need hospitalization or can we do it ambulatory. What are the criteria of rejecting human and veterinary patients, what we should offer and what to restrict for them? These basic questions should be handled by us at the level of governmental laws and prescriptions and the level of patient and owner information brochures as well.
- Work with open isotope sources while radiopharmacists, nuclear medicinists and technicians do radiopharmaceutical production, preparation, distribution and at Nuclear Medicine Departments in hospitals preparation of syringes, applicating the dose, imaging – the whole process must be safe and harmless for the specialists doing their job. Fullfilling this goal staff continously must be educated, steps of nuclear manipulations must be exercised with animal tests mimicing the clinical situations. A good example the sentinel lymph node detection in human oncological patients where all steps of isotope labelled agent preparation, application of radiopharmaceutical, scintiimaging (scanning), intraoperative detection as well as the pathological examinations of the nodes could be well evaluated and exercised by animal modelling. Experiences of animal tests could be provided for human and veterinary nuclear medicine specialists as local offers.
- Isotope wastes roduced by human or nuclear medicine applications are potencially dangerous for the environment and the earth population that is why handling of them needs special knowledge. These kind of wastes are never only pure isotope wastes but they are also mixed with biologically (excrets), physically (sharp blades, needles), chemically (medicines, solvents). Handling, minimalizing, short term storage and neutralizing of these dangerous wasted materials is an interdisciplinar special challenge for the specialists working in the field.
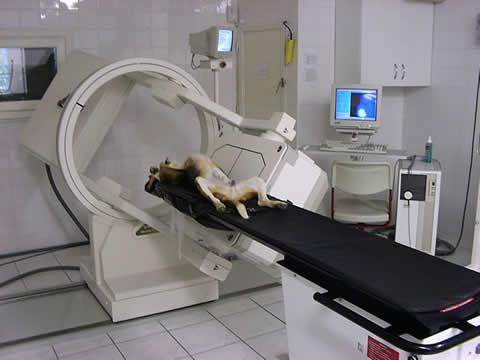
Fig. 1a.
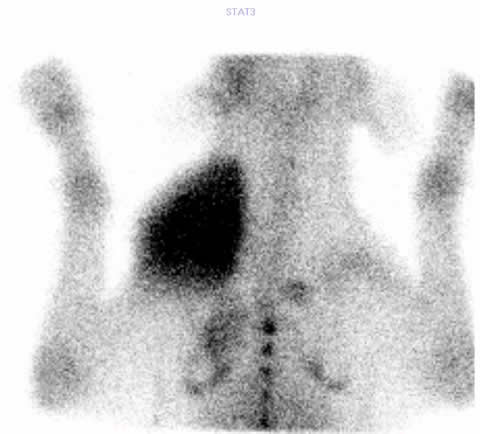
Fig. 1b.
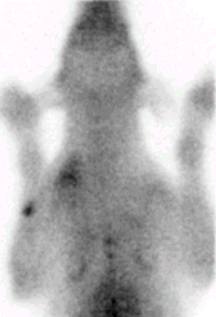
Fig. 1c.
In the photo there is an anaesthetized dog seen during scintigraphic examination (Fig 1a). By performing oncological scintigraphy efficacy of the therapy in malignant diseses could be well followed-up. In the Fig 1b one can see a huge inoperable tumor before treatment, and in Fig 1c the posttreatment small-sized, operable malignancy is seen.
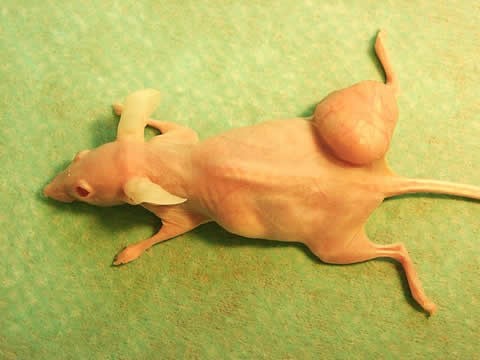
Fig. 2a.
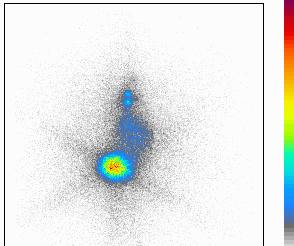
Fig. 2b.
In Fig 2a there is a immunosuppressed so-called Nude mouse is seen, in the right thigh region a transplanted human gastrointestinal cancer is growing. This modell is very much available for testing the availabness, effectivity and harmless of radiopharmaceuticals. In Fig 2b a scintigraphic image of the same mouse is presented, the suspected efficacy of the radiopharmaceutical (131J-anti CEA MoAb) in human malignant diseases is well-demonstrated in the scan.
Revision: 05-07-2007





